Section 3: Thermodynamics, or why do things happen the way they do?
Balanced chemical reactions are the math of chemistry
- They show the relationship between the reactants and the products
How will chemical reactions proceed?
- Thermodynamics allows us to calculate the feasibility of reactions and to understand when/how equilibrium is established
Equilibrium
- Allows us to understand chemical processes such as ionic speciation, oxidation state distributions gas solubility, the carbonate system
Balancing equations, oxidation state, redox
Equation balancing:
Examples: If a solution of lead nitrate is added to a solution of sodium chloride, lead chloride precipitates:
Conventional equation:
An ionic equation is written to show the actual species in solution:
This equation contains spectators that are not interacting, we can eliminate those spectators to get a net ionic equation:
Steps in writing a net ionic equation:
- Write the conventional equation, including designations of state [(g), (l), (s), (aq)]. Balance the equation.
- Write the ionic equation by replacing each dissolved substance (aq) with the species in solution. Never change states in this step. Be sure the equation is balanced for both atoms and charge.
- Write the net ionic equation by removing the spectators. Reduce coefficients to lowest terms. Be sure the equation is balanced for both atoms and charge.
Chemical Equilibrium
How do we know if a reaction will proceed?
- Is a geochemical system at chemical equilibrium?
- If not, what reactions are most likely to occur?
Types of processes where we would want to know if a reaction will proceed: Solubility - diatoms in surface seawater Redox - organic matter oxidation Complexation - iron speciation & plankton growth Carbonate system - CaCO3 stability in marine sediments
Consider a reversible reaction taking place at constant temperature:
The reactants A and B combine to form products C and D.
The concentrations of A and B decrease until they reach values that do not change with time:
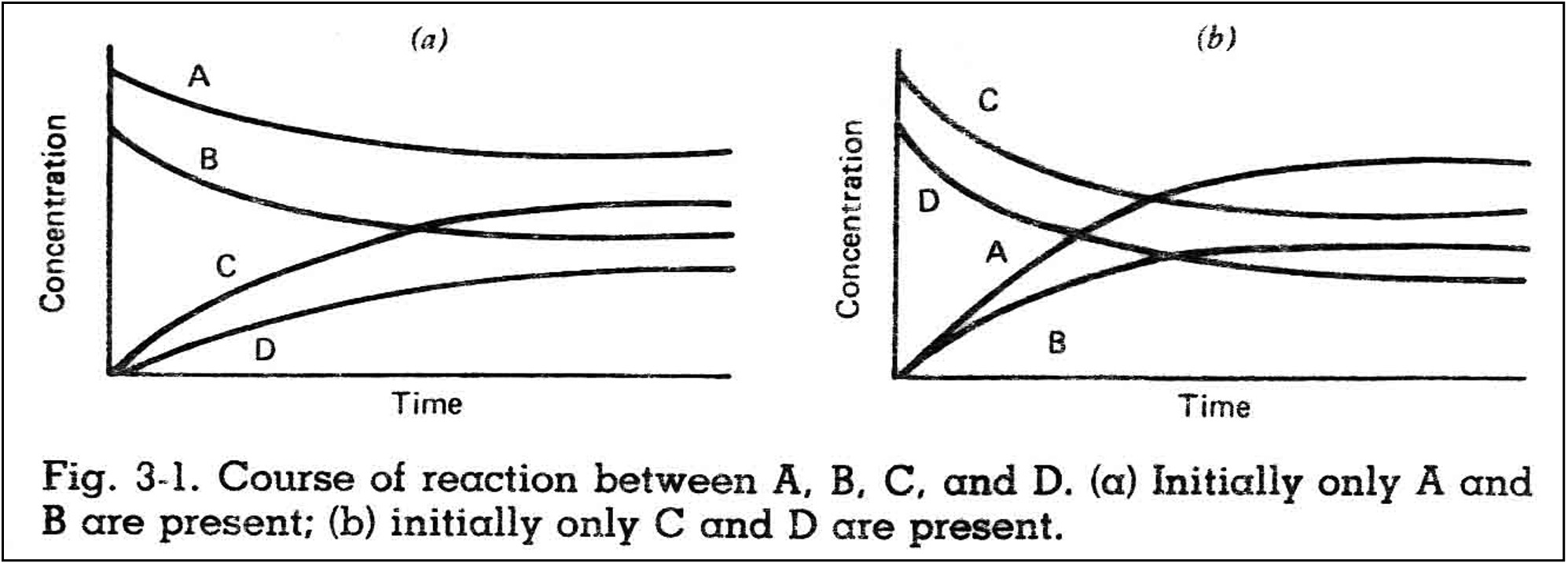
The time-invariant concentrations of reactants and products are called equilibrium concentrations. The ratio of these concentrations (or activities – active concentrations) is characteristic for each reaction, and is called the equilibrium constant, K:
Other than adding reactants and waiting until concentrations no longer change, how do we know what the equilibrium is?
Thermodynamics
…or how to predict chemical reactions without doing experiments
We want to answer these questions:
- Will this reaction happen?
- If so, how far can it proceed?
- We’ll only review a small subset of thermodynamics:
- Laws of Thermodynamics and how they apply to chemistry
- Entropy, Enthalpy
- Equilibrium
- Using Gibbs free energy to determine if a reaction will proceed as written
Laws of Thermodynamics:
- Conservation of energy – total energy remains constant (but can be converted from one form to another)
- Entropy (disorder) tends to increase
- Entropy is constant when the temperature equals absolute zero
Definitions:
- Enthalpy – total energy of an element or compound
- Entropy – degree of disorder (highly structured = low entropy, randomized = high entropy)
- Gibbs free energy – the part of the total energy available to perform “useful” work
Changes in enthalpy and entropy allows prediction of the feasibility of reactions
Reduction-Oxidation Reactions
Chemistry in Seawater: Redox potential, pE-pH
Acids and Bases + Carbonate chemistry
- Carbonate Chemistry class video:
Estuarine and coastal biogeochemistry
- Estuaries and coastal bgc video:
Diagenesis in sediments
Trace Metals (not on exam 2)
- Trace metals video:
- Exam 2 review video: